Modern sharks (505 spp.) [Sealchimorpha] and rays (350 spp.), and skates (293 spp.) [Batomorphii], which together form the Elasmobranchii, are one of evolution's most outstanding success stories with a fossil record ranging back at least to the Early Permian (295 million years ago), based on an isolated tooth of a supposed stem-group elasmobranch, †Synechodus aniquus (†Synechodontiformes) making them probably the oldest surviving vertebrate group. During their long evolutionary history, elasmobranchs survived several mass and minor extinction events and adapted to a wide range of aquatic environments including the open and deep-sea and even freshwaters. Today, they occupy the positions of top- and apex predators in food webs maintaining the balance and stability of ocean's ecosystems and contributing to the regulation of food webs. Unfortunately, our planet's biodiversity is experiencing nowadays an anthropogenically driven extinction, unlike any past mass extinctions, with global extinction rates elevated up to a thousand times. Despite being well-adapted predators, this new mass extinction event will not spare elasmobranchs with an estimated one-third of all species being threatened according to the IUCN Red List. The disappearance of elasmobranchs could have tremendous and catastrophic impacts on marine environments and thus on one of the major natural resources for humans as results of negative feedbacks on food web structures leading to ecological instabilities.
Although conservation efforts, as well as our knowledge of elasmobranch biology significantly increased in the last decades, there are still huge gaps to be filled to ensure the survival of this group. For this to be achieved, a solid taxonomic understanding of both extinct and extant elasmobranchs, well-resolved phylogenetic interrelationships and an understanding of their evolutionary history by including fossils play an increasingly important role in conservation biology. This enables to provide unique perspectives to the evolutionary history of a group and thus helps formulating priorities on how to maintain crucial diversities that are important for ecosystem stability and sustain vital natural resources for humans. Unfortunately, the few attempts to establish robust phylogenetic trees based on both fossil and extant taxa using morphological characters provided only very restricted and mostly ambiguous results. Recently, members of our team made a huge step forward in providing the first conclusive phylogenetic hypothesis based on morphological characters that unambiguously identifies batomorphs as sister group of selachimorphs, as molecular studies previously hypothesized. However, the inter- and intra-relationships of many taxa and even groups continue to be disputable.
Likewise, the origin of modern elasmobranchs for which identifying their stem-group is crucial, still is befogged preventing a deeper understanding of elasmobranch evolutionary history in deep-time. It is well established that stem- and crown groups influence each other with the stem-group generally diversifying rapidly until the crown group emerges, resulting in its diversity collapse and subsequent extinction. Previous works showed that mass extinctions, might disturb this pattern creating long stem-groups (e.g., dinosaurs) and that crown groups usually do not emerge cryptically or just before mass extinctions, in contradiction to popular hypotheses. Consequently, the evolutionary history of elasmobranch fishes can be divided into three sequential phases:
1st: Presence of stem representatives providing vital information about the emergence of the group
2nd: Co-occurrence of stem- and crown groups
3rd: Only crown groups present
In addition to the 1st phase, the 2nd phase is of major interest here, because the length of overlap between both groups might depend on significant drivers (e.g., extinction events, niche portioning, competition). Thus, the stem-group might influence the emergence of modern groups, which, however, never has been tested.
The fundamental goals of this project are to gain novel and deeper insights into patterns and processes of elasmobranch evolution and diversities in deep-time, and mechanisms underlying the related processes, morphological novelties and the success and demise of elasmobranchs.
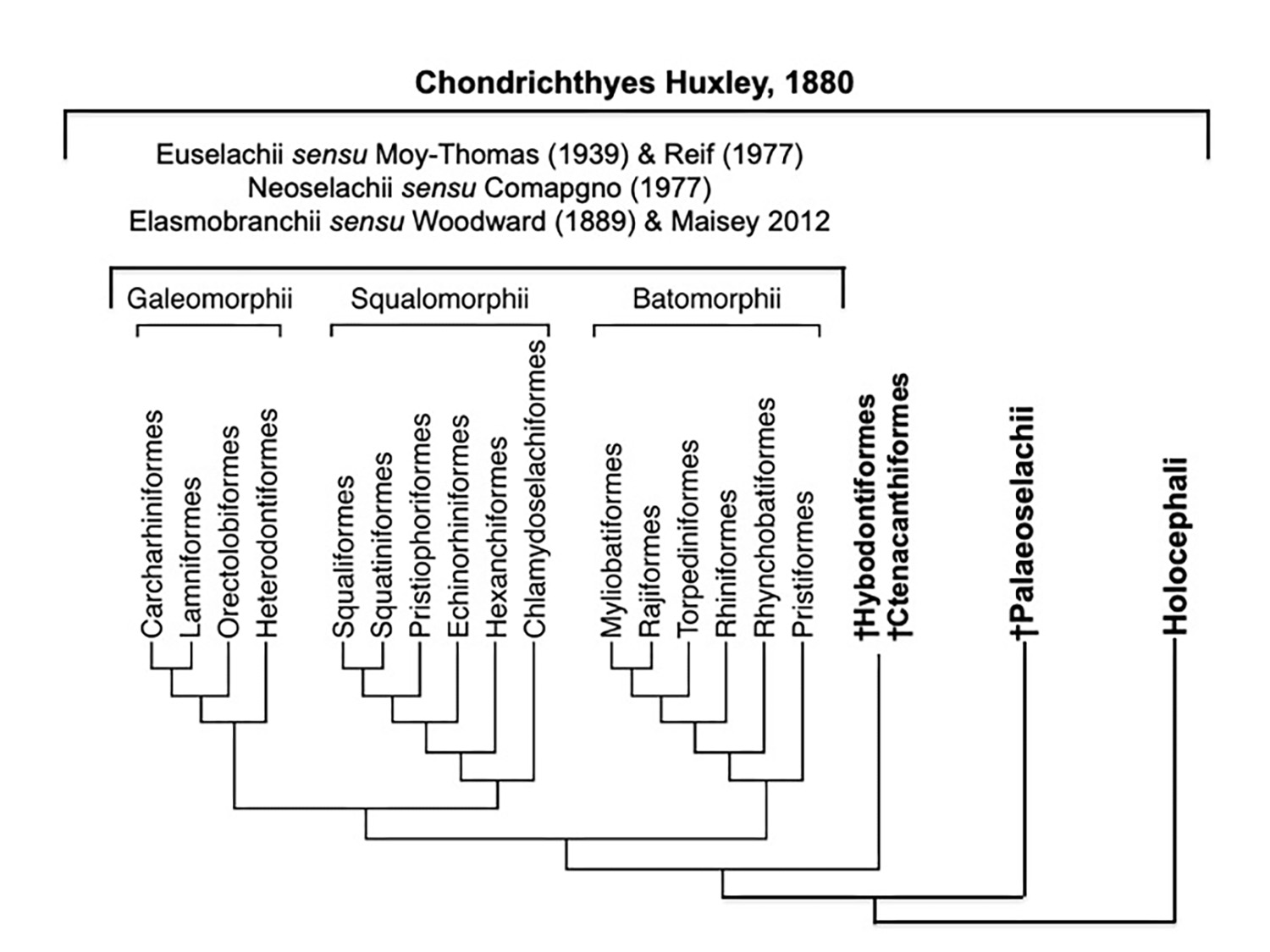
Hypothesized interrelationships of Chondrichthyes. (unpublished)
up |
|
|
|
1.1 Origin and early evolution of elasmobranchs |
Background & Goals:
| Many researchers have proposed hypotheses about the origin and relationships of fossil and extant elasmobranchs dating back into the 19th century. Today, the monophyly of extant elasmobranchs is widely accepted and has been confirmed by molecular and morphological data, but the phylogenetic relationships of various elasmobranch groups, especially extinct clades, still is ambiguous despite all recent progress. But the fossil record of elasmobranchs is far from being poor as often assumed, even though skeletal elements normally only fossilize under particular taphonomic conditions. Consequently, a plethora of extinct taxa has been described mostly based on isolated teeth since the first attempts to include fossils into a larger framework of elasmobranch taxonomy and systematics. This emphasizes the importance of isolated teeth for identifying extinct but even extant taxa. However, dental morphologies of most extant elasmobranchs still are not well established forming a continuous problem to establish stable alpha taxonomies. Consequently, teeth are assumed to only provide limited data sets for phylogenetic analyses and only a few studies used exclusively dental characters for phylogenetic inferences. As a result, most fossil, especially pre-Jurassic elasmobranchs have uncertain phylogenetic positions and the dominance of isolated teeth in their fossil record renders recognition of evolutionary events difficult. Nevertheless, teeth bear not only taxonomic and functional but also phylogenetic signals.
The supposedly oldest known elasmobranch remain belonging to a synechodontiform (see also above) comes from the Early Permian (295 Ma). The monophyly, taxonomic composition and identification of †Synechodontiformes (Permian-Palaeogene; including complete skeletons) as stem elasmobranchs, however, is still controversially discussed. The appearance of hybodontiforms (the sister group of elasmobranchs) in the Devonian suggests that elasmobranchs also originated in the Devonian, which would be in accordance with the Devonian occurrence of the enigmatic shark †Mcmurdodus (teeth, rare postcranial remains including ring-like structures that most likely surrounded the lateral line system). Possible other stem elasmobranchs include †Cooleyella and †Ginteri (Carboniferous, teeth only), †Doratodus, †Duffinselache, †Pseudodalatias, †Rhomaleodus and †Vallisia (all Triassic, teeth only) However, it is currently difficult to resolve the systematic position of all these putative stem elasmobranchs because the phylogenetic signal of their dental traits has not yet been established. The origin of crown (modern) elasmobranchs is considered to have occurred in the Triassic. The morphological variety of Triassic and Early Jurassic elasmobranchs implies that both stem and crown group members were probably far more diverse during this time interval than previously appreciated. During the Rhaetian transgression (Late Triassic), which created extensive shallow epicontinental seas, major radiations and diversifications of elasmobranchs occurred that probably all represent stem members. After the Late Triassic mass extinction, crown elasmobranchs rapidly diversified and expanded in the Early Jurassic, with the first appearance of the three crown elasmobranch clades (Galeo-, Squalo-, Batomorphii). The post-Triassic record of stem elasmobranchs remains very ambiguous and therefore does not allow any assumptions about their diversity and extinction patterns and possible interactions with crown elasmobranchs. Moreover, although skeletal remains of fossil elasmobranchs have been increasingly included in phylogenetic analyses in the last 30 years, the overwhelming majority of these taxa represent crown group members, leaving the taxonomic and phylogenetic affinities of most pre-Cretaceous taxa unresolved. This results in numerous taxonomic and systematic issues for extinct elasmobranchs (e.g., unclear interrelationships, impact on evolution of crown group) and demonstrates our still inadequate understanding of the origin and early evolution of elasmobranchs.
However, how to identify stem (basal) group members if the fossil record of elasmobranchs predominantly consists of isolated teeth? Traditionally, fossil elasmobranch and non-elasmobranch teeth were distinguished using the histology of the superficial, hypermineralized tooth enameloid since the pioneering works of W.-E. Reif in the 1970s. Accordingly, elasmobranchs teeth are characterized by a triple-layered enameloid consisting of a single-crystallite (SCE), parallel bundled (PBE), and tangled bundled layer (TBE), which is reduced in batoidimorpha and also in molariform teeth of some sharks, e.g., bullhead sharks. The traditional approach, however, appears to be oversimplified and the enameloid structures found in non-holocephalan chondrichthyans seemingly are more varied than previously assumed. This finding resulted in a modified scheme, in which the generalized enameloid consists of two units, the SCE and BCE (bundled crystallite enameloid), where the BCE comprises three units (TBE, RBE [radial bundled enameloid], PBE). This pattern obviously is present in all sharks but varies in all other elasmobranchs11. The microstructure of putative Palaeozoic stem elasmobranchs displays very distinct enameloid microstructures with either having none or only one layer, while that of hybodontiforms typically has two layers (SCE, RBE). However, the discovery of Early Cretaceous elasmobranch teeth with Palaeozoic cladodontomorph tooth morphologies but with the presence of a PBE layer (considered an elasmobranch synapomorphy) complicates things further. This brief review exemplifies the confusing and not-well understood distribution of enameloid microstructures in non-holocephalan chondrichthyans even though the enameloid microstructure seemingly also bears phylogenetic signals. Additionally, it also was demonstrated that the underlying tooth dentine has the potential to bear phylogenetic signals.
In this project, we plan to combine complete skeletons and dental morphologies and histologies, providing not only a unique evolutionary perspective of the first 250 million years of elasmobranch evolution, but will represent the most integrative study on phylogenetic relationships of elasmobranchs. This will provide vital information beyond our current knowledge by providing fresh perspectives and additional methodological tools to place morphological findings into a phylogenetic context.
Major specific research questions of this project are: (1) Which elasmobranchs are stem-group representatives? (2) Where are they positioned on the stem of Elasmobranchii, and what is their sequence of appearance and disappearance? (3) What is the timing, mode and tempo of morphological trait and phenotypic evolution within stem elasmobranchs? (4) What is the phylogenetic relevance of tooth morphologies and histotypes in stem elasmobranch phylogeny? (5) What are the morphological differences between stem and crown elasmobranchs? (6) Was stem and crown elasmobranch evolution interdependent?
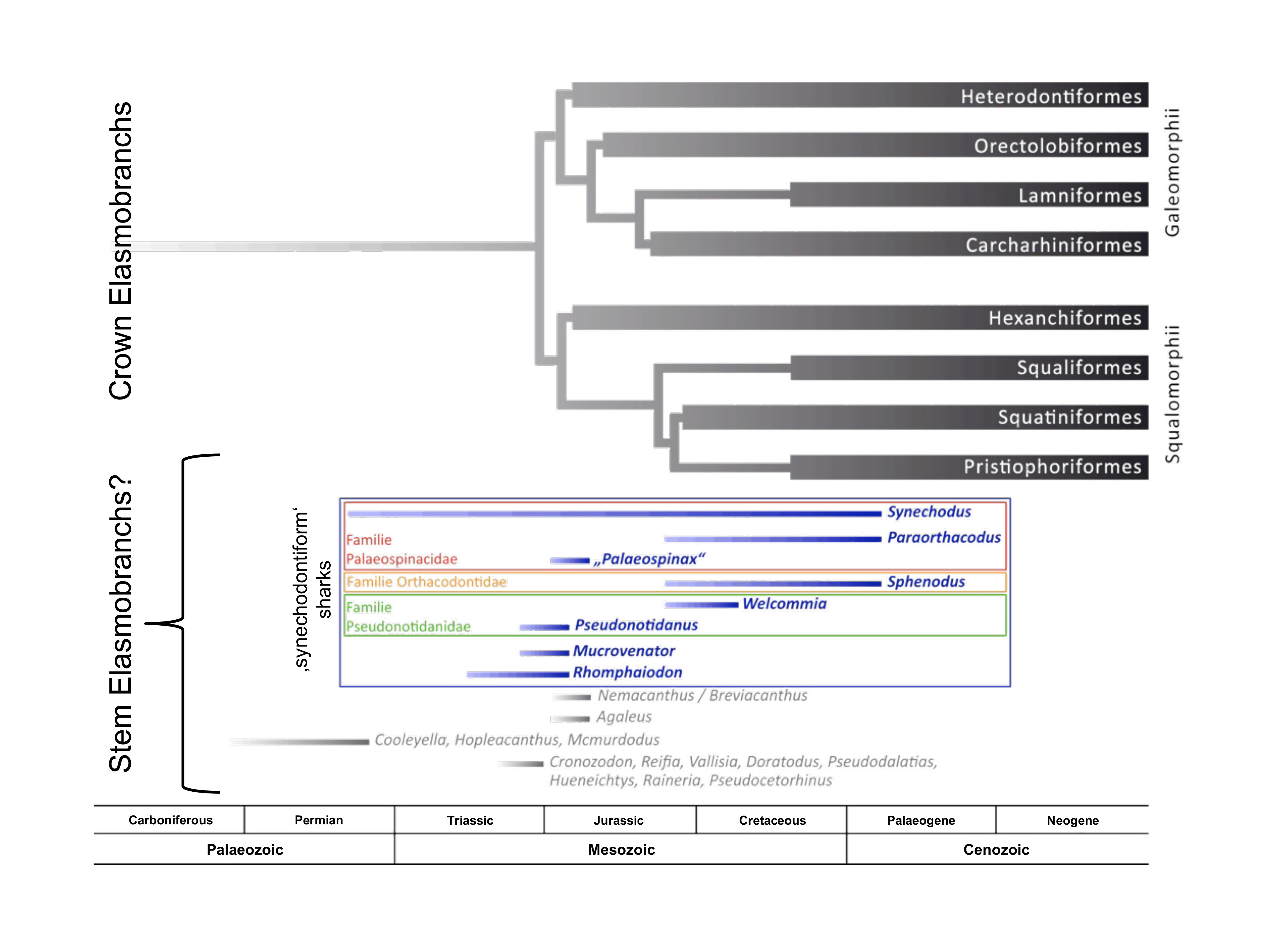
Crown elasmobranchs and candidate stem elasmobranchs. (unpublished)
| up |
|
|
|
1.2 Origin and evolutionary history of crown elasmobranchs |
Background & Goals:
| Crown elasmobranchs (sharks, rays, and skates) form together with the rat fishes (Holocephali) the last extant members of the class Chondrichthyes, which represents the sister group to all other living jawed vertebrates. This very successful group has survived all five mass and several minor extinctions. The monophyly of elasmobranchs is well supported by both molecular and morphological data. Morphological synapomorphies for this group include a triple layered tooth enameloid, a segmented, calcified notochordal sheath, a metapterygium that spans the entire pelvic fin, one or two intermediate pelvic clasper cartilages and an anterior dorsal fin with a complete calcified radial series. Another feature present in all elasmobranchs (but not exclusive to them) are several rows of highly specialized teeth and a lifelong tooth replacement (polyphyodont dentition), which allowed them to occupy a wide range of different ecological niches at the top of aquatic food webs. In modern but also past marine environments, elasmobranchs occupy upper trophic levels and represent meso- and top predators.
As for stem elasmobranchs, isolated teeth predominantly also characterize the fossil record of crown elasmobranchs, which renders recognizing the timing of their origin difficult. The origin of modern crown elasmobranchs is considered by many researchers to have occurred in the Triassic. However, this is based on dental morphologies and the taxonomic identity of the corresponding producers. No comprehensive phylogenetic analyses employing strict cladistic principles have been conducted so far to reconstruct the origin of crown elasmobranchs, let alone to understand the inter- and intra-relationships of both extinct (fossil) and extant taxa.
Many researchers proposed hypotheses about the relationships of fossil and extant elasmobranchs, the oldest of which date back into the 19th century. Today, the monophyly of extant sharks, rays, and skates is widely accepted and has been confirmed by both morphological and molecular data, but the phylogenetic relationships of various crown elasmobranch groups still remain ambiguous despite all progress that has been accomplished in recent years.
Since the first attempts to include fossils into a larger framework of elasmobranch taxonomy and systematics, a plethora of new taxa have been described mostly based on isolated teeth. This emphasizes the importance of isolated teeth for identifying extinct but even extant taxa. However, the dental morphologies of most extant elasmobranchs still are not established, which forms a continuous problem to establish solid alpha taxonomies. Teeth, however, not only bear taxonomic and functional signals but also phylogenetic information. Nevertheless, teeth only provide a limited data set for phylogenetic analyses and only few studies used exclusively dental characters for phylogenetic inferences that showed clear limitations in using such characters alone for phylogenetic reconstructions. Additional cranial and postcranial characters, therefore, are mandatory for better understanding the systematic position of extinct taxa and the evolution of traits. The fossil record of crown elasmobranchs actually is far from being poor as often assumed, even though the skeletal elements normally only fossilize under very specific taphonomic conditions. Articulated skeletons are known from all major groups and most geological epochs as exemplified by an increasing body of published records. However, fossil crown elasmobranchs only rarely were included in phylogenetic analyses, which certainly is related to the nature of preservation of fossil taxa. Interestingly, most available studies include batoids such as Late Jurassic, Early and Late Cretaceous, and Eocene batoids. Sharks, conversely, only rarely were included in phylogenetic analyses, The lack of a comprehensive understanding of interrelationships of crown elasmobranchs, which still continues, both extinct and extant also prevents detailed analyses about character evolution and thus to reconstruct their evolutionary history from a morphological perspective employing robust analytical procedures.
The ultimate goal of this research topic therefore is to augment our understanding of the evolution, tempo, and timing of morphological traits, and phenotypic appearances in modern elasmobranch fishes (sharks, rays, skates), to identify correlated and possible times of enhanced or increased phenotypic changes, or whether character evolution continued regularly.
To achieve this, we will: (1) clarify the systematic and phylogenetic interrelationships of extinct elasmobranch fishes based on cranial and postcranial material, with the goal to establish a robust phylogenetic tree based on a combination of molecular, morphological and fossil data, (2) date the origin and divergences of the various clades, (3) analyze the timing of origin, divergence, and evolutionary pathways of skeletal characters and body plans.
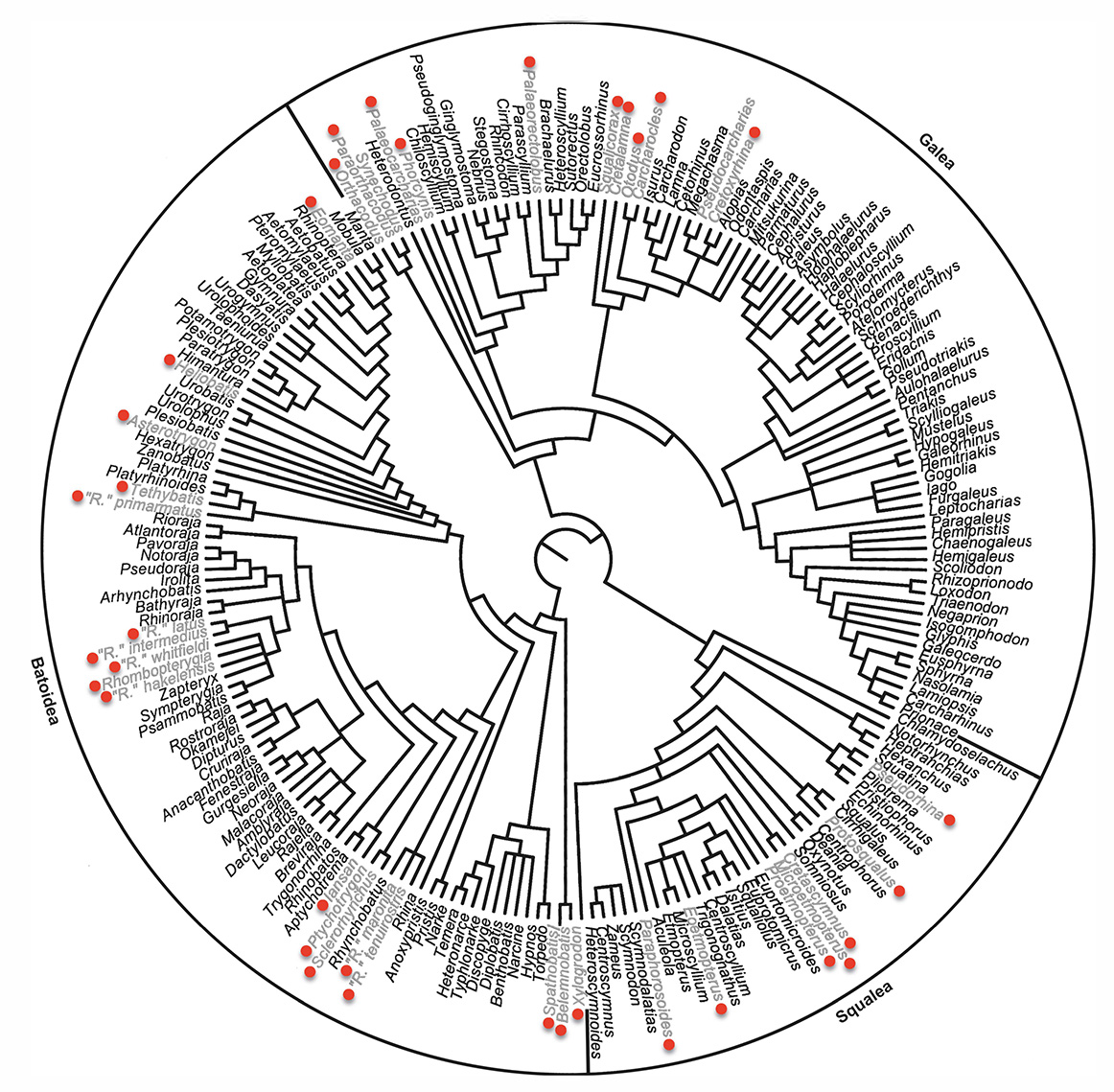
A supertree of crown elasmobranchs including fossil taxa indicated by red dots. (unpublished)
| up |
|
|
|
1.3 Evolutionary developmental morphology of cartilaginous fishes |
Background & Goals:
| This research area combines the traditional domain of palaeobiology with developmental morphology and developmental genetics. This project focus on the early ontogenetic development of chondrichthyan fishes, which constitute one of the two major crown groups of gnathostomes with an evolutionary history ranging back some 420 million years (probably even far more when isolated fragments are considered). The focus currently is on elasmobranch fishes (sharks, skates, rays) that become more and more model organisms for understanding the development of gnathostome traits. Here, we intend to identify phylotypic stages (= point of time when embryos of one species resemble those of others) across extant chondrichthyan clades during embryological development to provide detailed staging sequences for different clades. Timing shifts during the development of different species are well known but not yet established. These shifts in the developmental timing of organs and skeletal structures denote developmental autonomy and signify heterochronic developmental patterns. Heterochrony is a fundamental concept in macroevolutionary research. It describes time shifts of developmental events, which changed during evolution. Here, concepts and methodological approaches of morphological integration (= tendancy of different traits to vary jointly) and modularity (= modules characterized by concentration of integrated morphological traits) by employing different approaches such as Geometric Morphometrics, matrix correlations, and principal component and principal coordinate analyses but also developmental genetic approaches will be combined. This research topic thus focuses on the connection of developmental morphological trait evolution within a phylogenetic framework of cartilaginous fishes to better understand processes underlying diversification and taxonomic diversity patterns through time. The relationships between early developmental and evolutionary character transformation still also is one of the major controversies in palaeobiology since the times of E. Haeckel (1866), who proposed the recapitulation theory. Thus, we seek to better understand, how organs develop during early ontogeny and how this developmental traits relate to macroevolutionary patterns.

Embryos, juvenile and adult specimens of Chlamydoselachus anguineus (Tokkai University, Shimizu, Japan) [© J. Kriwet].
| |
up |
|
|
|
| 2. Actinopterygii: Evolution and adaptations |
Background & Goals:
| MMMMMM
| up |
|
|
|
Evolutionary history and ecological adaptations of pycnodontiform fishes |
Background & Goals:
| Pycnodont fishes (Pycnodontiformes) are a monophyletic and ecologically successful clade of extinct ray finned fishes with a fossil record spanning 175 million years from the Late Triassic to middle Eocene. They are most commonly associated with shallow water marine habitats but are also found in association with freshwater and estuarine deposits. A deep, rounded, and laterally compressed body, a frontal flexure of the skull in profile view, a more or less prognathous snout, and elongated dorsal and anal fins, forming together with the caudal fin an effective rudder characterize pycnodonts. In their body shape they superficially resemble extant coral reef fishes like butterflyfishes (Chaetodontidae), doctorfishes (Acanthuridae), and triggerfishes (Balistidae). Distinctive characteristics of pycnodonts are their molariform teeth, which generally are arranged in well-defined rows on the unpaired vomer (upper jaw) and paired prearticulars (lower jaws). This dentition represents an adaptation to shelled prey. Their diversity is strikingly high, with over >1000 nominal species in >55 genera of which many taxa are based on teeth and dentitions only. The palaeobiogeography of these fishes suggests they originated in the Tethys Sea initially and then spread worldwide.
Traditionally considered an order, Pycnodontiformes, they are now accepted to represent a superorder, Pycnodontomorpha, which was considered as a possible sister group candidate to Teleostei (Nursall 2010). However, a more recent analysis identified pycnodonts to be the sister group to Halecostomi (Holostei + Teleosteomorpha), which would make pycnodonts the most basal group among neopterygian fishes. Pycnodontomorpha comprises the orders Gyrodontiformes containing the families Gyrodontidae and Mesturidae, and Pycnodontiformes, including all remaining taxa. In this application whenever the term pycnodont is used, it refers to the total group Pycnodontiformes.
The fact that pycnodonts weathered both the Late Triassic/Early Jurassic and the K/Pg extinction events indicates that these fishes had a particularly successful evolutionary strategy. Indeed, their increasing raw species diversity through the Mesozoic is quite plainly seen in the fossil record (Late Triassic: 4; Early Jurassic: 3; Middle Jurassic: ca. 16; Late Jurassic: ca. 77; Early Cretaceous: ca. 73; Late Cretaceous: >10 [only named and currently as valid considered species]). The increase in raw species numbers in the Late Cretaceous is mirrored in the great increase in morphological disparity. The increase in species diversity from the Middle Jurassic onwards indicates the first diversification event in the history of pycnodonts.
A steep drop in taxon number occurred from the Late Cretaceous to Palaeocene, which might indicate that the K/P boundary event also affected pycnodont fishes. The disappearance of most major lineages well before the K/P boundary might be related to the so-called Signor-Lipps effect. Most pycnodonts known from the Palaeocene also occur in the Late Cretaceous with the exception of members of Pycnodus, which seemingly originated in the Palaeogene. This pycnodont is the most derived member of Pycnodontomorpha and the last representative of this formerly diverse group in the Eocene before it vanished, too.
A popular hypothesis regarding the extinction of pycnodonts is the observation that many modern teleost groups common in coral reefs today arose at the same time that pycnodonts were beginning to decline. Were the pycnodonts simply outcompeted by better-adapted teleosts or could there have been other factors in their extinction? But the reasons for the disappearance of pycnodonts might have been more complex as recent studies of our group showed.
While the monophyly of pycnodontiforms fishes is well supported, their intra-relationships, but also relationships to other actinopterygians have been discussed controversial for a long time and no general agreement has been achieved up to now. This even more complicated by numerous publications describing mainly new taxa from the Upper Cretaceous of Lebanon, but without providing any phylogenetic analyses employing robust cladistic principles to support the corresponding interpretations.
This project focuses therefore on the (1) dental, cranial and postcranial morphology, (2) taxonomy and systematics (phylogenetics), (3) evolution, (4) functional aspects of the feeding apparatus, (5) ecology, and (6) palaeobiogeography of pycnodontiform fishes.
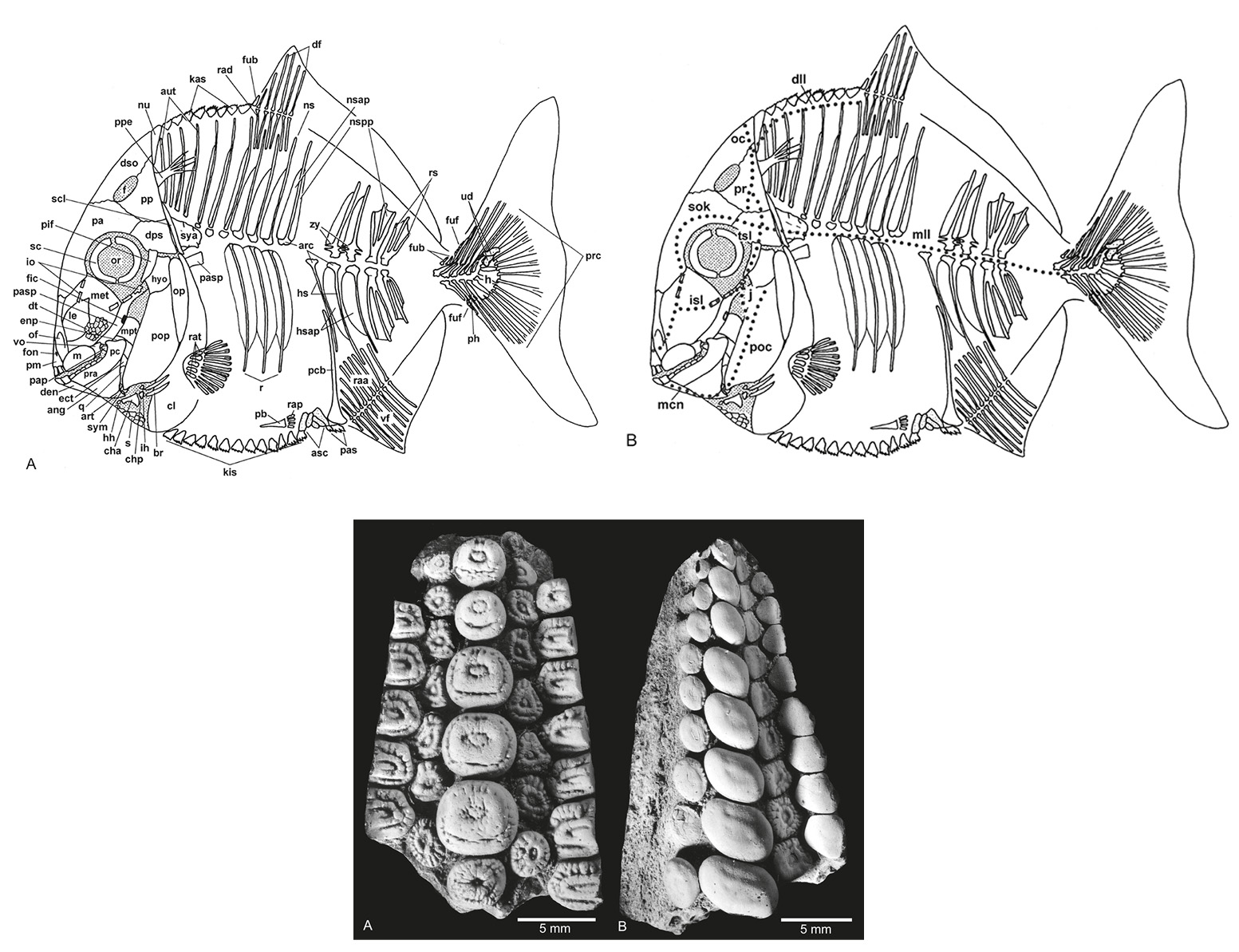
Above: Composite pycnodont displaying the general pycnodont characters. A: Bony elements. B: Sensory lines. Not to scale.
Below: Isolated dentitions of Gyrodus "planidens" (MB. f. 7173) from the Upper Jurassic (Tithonian) of Weymouth, UK. A: Vomerine dentition. B: Right prearticular dentition. (Modified from Kriwet, J. 2005. A comprehensive study of the skull and dentition of pycnodont fishes (Neopterygii, Pycnodontiformes. Zitteliana, vol. 45.)
| up |
|
|
|
Evolution and Adaptation of Fishes to the Deep-Sea |
Background & Goals:
| The adaptation of fishes to deep-sea conditions is another emerging topic. Generally, it is assumed that the modern deep-sea benthic fish fauna originated in shallow waters and subsequently replaced ancient deep-sea assemblages, which became eliminated by mass extinction events. This indicates that extinction events are the main trigger for deep-sea colonization of fishes. It also is generally assumed that plesiomorphic ray-finned fish groups have more deep-sea representatives than derived ones. This study, however, is based on maximum depth occurrence data of living forms, which might be prone to error as these authors indicate. The timing and underlying processes / reasons remain ambiguous. Moreover, no definition for deep-sea fishes currently exists. Here, we intend to identify key innovations in the musculoskeletal system and to establish morphological traits for identifying deep-sea fishes. This is essential for reconstructing evolutionary traits and pathways of modern deep-sea fishes. Additionally, reliable fossil record analyses for dating such adaptive events but also diversifications in the deep will be developed and employed for providing alternative hypotheses to those currently based on molecular clocks.
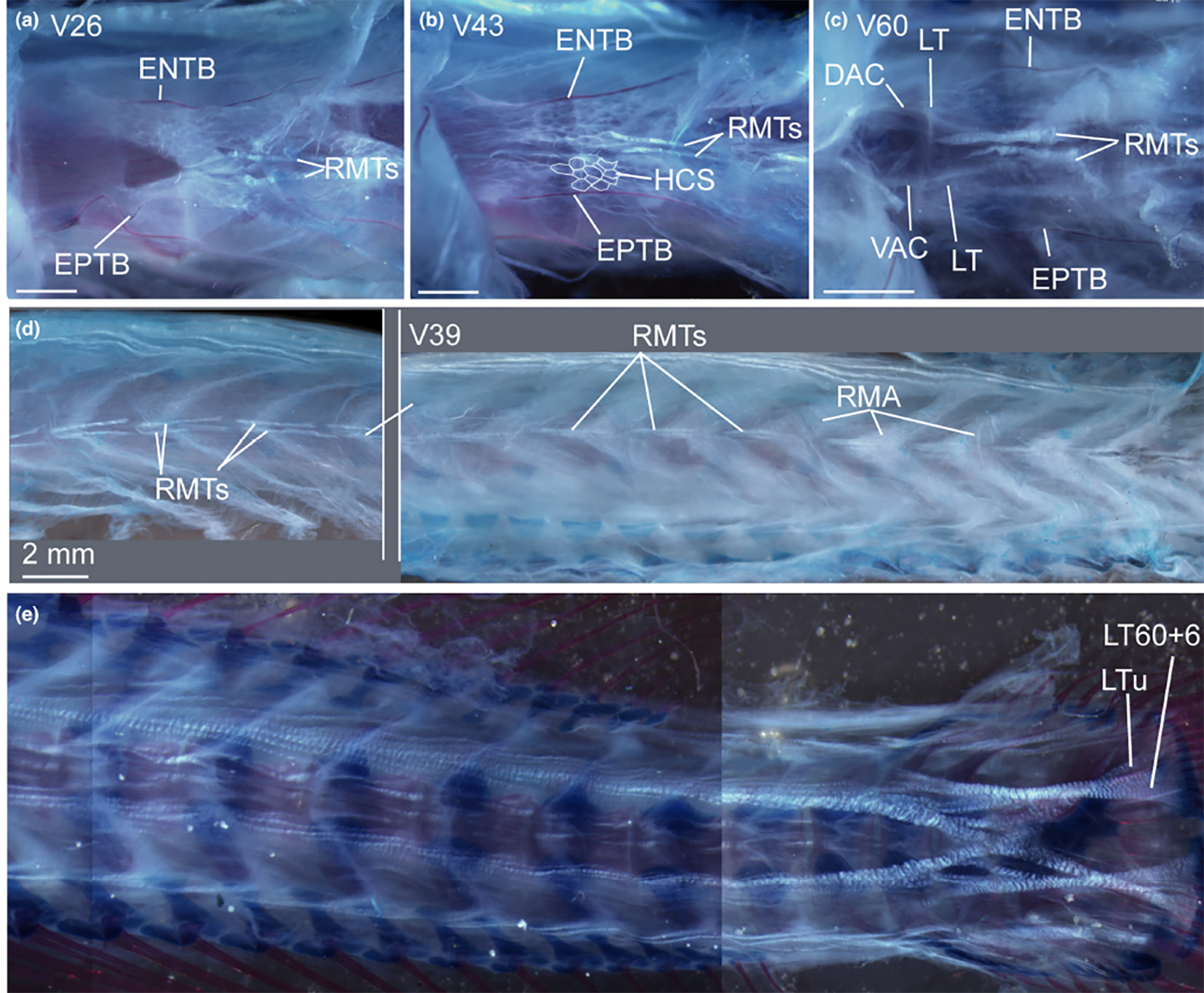
Cleared and double stained specimen of Eustomias obscurus (USNM 206711, 199 mm SL) under polarized light to show myosepta and tendons. (From Schnell etal. 2021. The musculotendinous system of mesopelagic fishes, part I: Stomiiformes (Teleostei). Journal of Anatomy, vol. 240.)
| |
up |
|
|
|
| 3. Drivers and sustainers of biodiversity in deep-time, and conservation of fishes |
Background & Goals:
| MMMMMM
| up |
|
|
|
Triassic recovery of fishes |
Background & Goals:
| Recovery from extinctions and ecosystem building are key themes in conservation biology, but also in Earth sciences. Extinctions of species are important in maintaining and changing the Earth's biodiversity. Extinction events result in a drastic reduction in the quantity of life on Earth and affect biodiversity directly. Five mass extinctions spanning the Phanerozoic have been identified up to now, each resulting in a loss of ca. 50% of all known species at the time. The end-Permian mass extinction 252 million years ago was the most severe and wiped out some 90% of marine species, and ecosystems were globally devastated. It is evident that the world after the end-Permian events remained largely devastated for the next 5 million years during the Early Triassic, causing delayed recoveries of life and normal ecosystem functioning at least until the Middle Triassic. However, recovery patterns of life during the Triassic still are incompletely known and understood despite all progress that has been accomplished in recent years, due to imprecise dating of fossil-bearing strata, uncertainties about the quality of the fossil record, and the lack of sound phylogenetic analyses of Triassic forms.
This project aims at understanding mass extinctions and recoveries in deep time, macoevolutionary trait evolution, the complex interplay of increasing species richness and morphological disparity, and fine-tuning of ecosystems during diversification. Here, rigorous numerical studies of ecology and evolution such as connectivity between ecosystem balance and recovery, trait evolution (e.g. body size, feeding apparatus, locomotory appendages), and disparity of fishes will be employed.
The ultimate goal of this project is to reveal (with ghost lineages) the timing, tempo and ecological nature of the recovery from the Permian-Triassic extinction event, and to test macroecological hypotheses. The integrated goals are to (1) complete documentation of Permian and Early and Middle Triassic marine fishes; (2) perform cladistic and composite phylogenies of relevant fish clades; (3) date these faunas accurately and integrate with the global-scale database on fish diversity through the Triassic; (4) analyse the evolution of different traits; (5) to explore disparity patterns; (6) establish local and global faunal relationships and endemism patterns of Triassic South China fishes; (7) analyse diversity and diversification patterns, and ecosystem dynamics, and, finally, (8) to integrate all results in a generalized model.
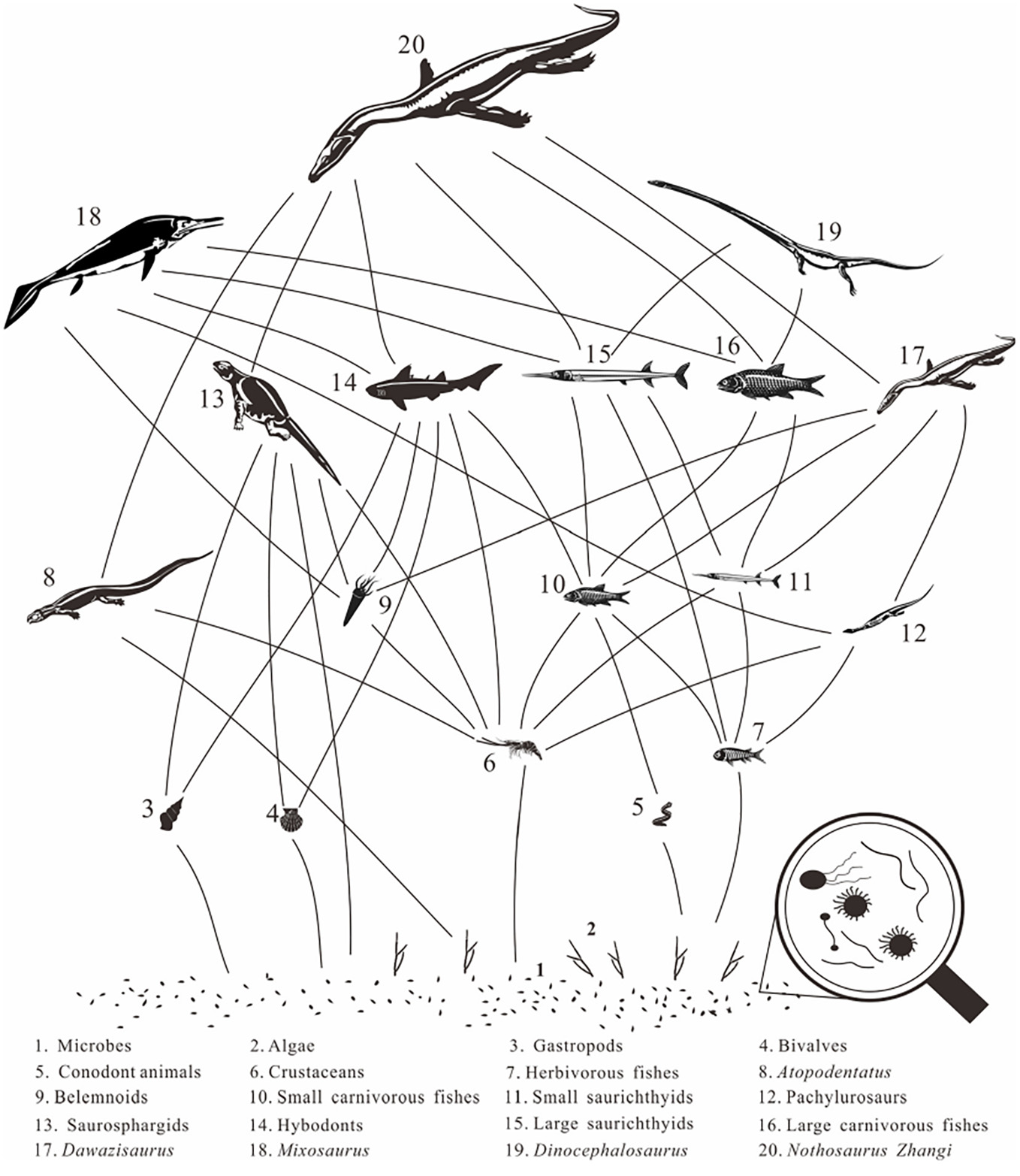
Hypothesized food web of the Anisian (Middle Triassic) Luoping Biota of China after the Eraly Triassic recovery. (From Wen et al. 2023. First occurrence of hybodontid teeth in the Luoping Biota (Middle Triassic, Anisian), emphasizing recovery of the marine ecosystem after the end-Permian mass extinction. Palaeogeography, Palaeoclimatology, Palaeoecology, vol. 617.)
| up |
|
|
|
Elasmobranch fishes (sharks, skates, and rays) of the Mediterranean Sea: Status and conservation needs |
Background & Goals:
| Elasmobranch (sharks, rays, and skates) populations are seriously endangered because of direct and indirect human impacts. As a consequence of intense exploitation, many species are listed in the Red List by the International Union for Conservation of Nature (IUCN) either as vulnerable or endangered. They are exceedingly vulnerable due to their low growth rates, late sexual maturi-ty and low fecundity.
One of the most overfished areas in the world is the Mediterranean Sea (FAO, 2018). At the same time it has one of the oldest histories of human-shark interactions due to ancient fishing communities. Today, scientists are fighting to keep track of shark and ray populations as they are declining at ever increasing speed. To protect the remaining elasmobranch biodiversity, it is of utmost importance to determine the baseline community: The taxa that used to live in the Mediterranean Sea before any anthropogenic impact on them. It is surprising how little we know about the taxonomic composition of the Mediterranean elasmobranch fauna and how large the individual populations are. Despite severe fishing pressures, the Mediterranean Sea still is regarded as a biodiversity hotspot due to a high percentage (20-30%) of endemic taxa (UN Environmental Program, 2023). This might be related to the Zanclean flood, which occurred 5.33 Ma, when today’s biota entered the Mediterranean Sea from Atlantic waters and found very different conditions and habitats: High salinity, higher temperatures and more stable conditions than in the Atlantic. This might have caused high levels of speciation and many cryptic species in the Mediterranean Sea might exist that still are waiting to be discovered.
Knowing the exact number of elasmobranch species and their distribution patterns across the Mediterranean Sea is crucial to inform the broader public, politicians and stakeholders about their extinction risk and necessary conservation measures.
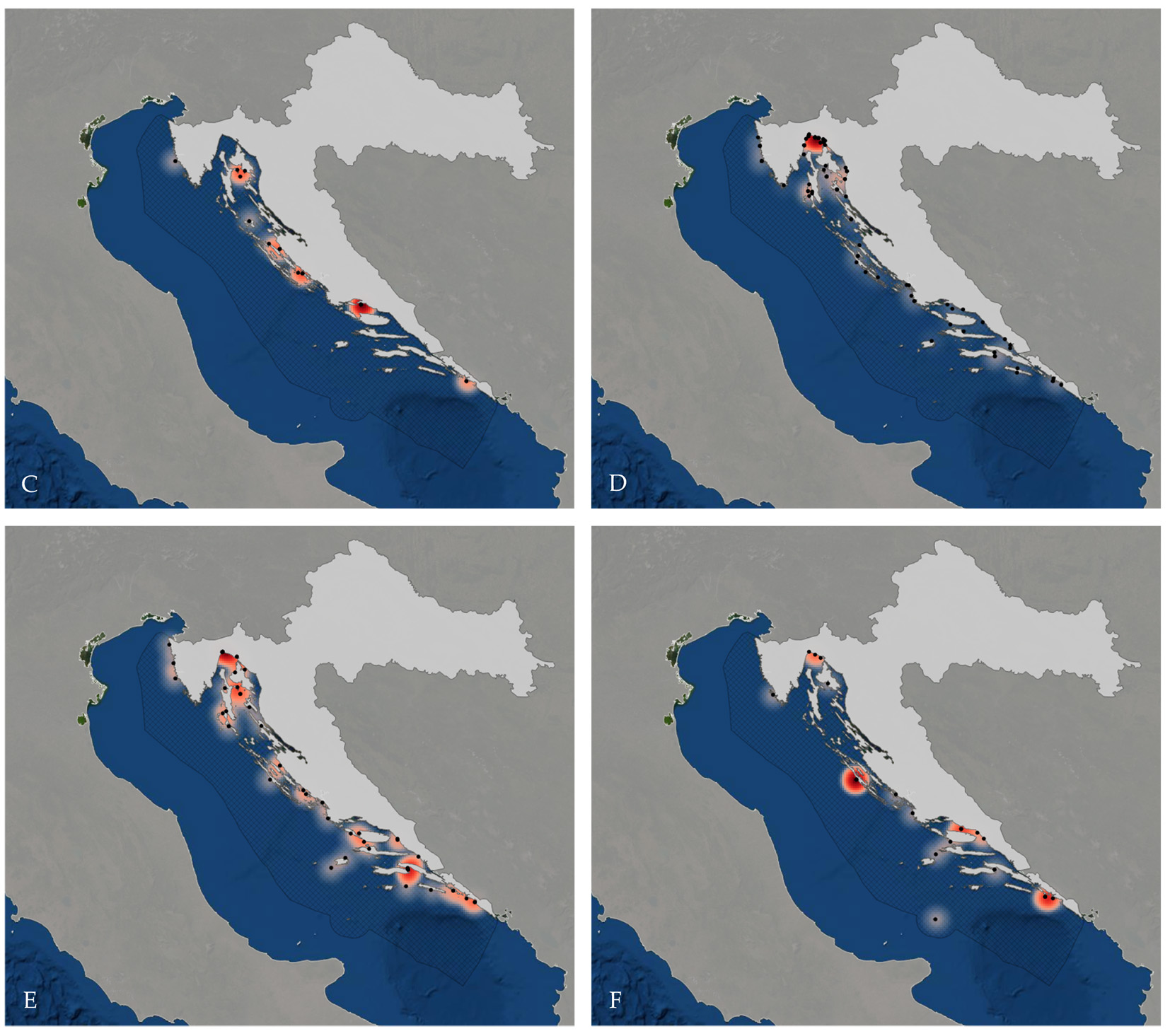
| Heatmap of all records where locations were provided (A), and species that are critically endangered in the Mediterranean, and have recent (2008-2021), and more than 10 records: Oxynotus centrina, (B), Squatina squatina (Linnaeus, 1758) (C), Carcharodon carcharias (Linnaeus, 1758) (D), Isurus oxyrinchus Rafinesque, 1810 (E), and Prionace glauca (Linnaeus, 1758) (F). (From Balàka, P. 2020. Updated Checklist of Chondrichthyan Species in the Croatian Sea. BSc Thesis, University of Vienna.)
| up |
|
|
|